Strength in Weakness: Slow Excitation Increases Temporal Precision of Sound Offset Encoding
Material below summarizes the article Slow NMDA-Mediated Excitation Accelerates Offset-Response Latencies Generated via a Post-Inhibitory Rebound Mechanism, published on May 31, 2019, in eNeuro and authored by Ezhilarasan Rajaram, Carina Kaltenbach, Matthew J. Fischl, Leander Mrowka, Olga Alexandrova, Benedikt Grothe, Matthias H. Hennig, and Conny Kopp-Scheinpflug.
Highlights
- In response to short sound stimuli in vivo, some superior paraolivary nucleus (SPN) neurons display only offset responses (OFF-only), while others display both onset and offset responses (ON-OFF).
- The response to the end of the acoustic stimulus in these ON-OFF neurons occurs significantly faster in comparison to OFF-only neurons and is surprisingly invariant to changes in sound intensity.
- The presence of strong inhibition and weak excitation in SPN neurons as identified by patch-clamp recordings was fed into a computational model, which indicated that NMDA receptors, though weakly expressed, play an important role in improving the temporal precision of the offset response.
 |
|
|
Study Question
A neuroscience textbook paints the neuron as receiving a good measure of excitatory and inhibitory inputs. Strong excitatory inputs depolarize the neuron to power an action potential, while inhibitory inputs hold the cell back by hyperpolarization.
The neurons of the superior paraolivary nucleus (SPN) in the auditory brainstem of rodents embody the polar opposite. They receive predominantly inhibitory inputs. It has been shown that SPN neurons rely on strong inhibition to fire action potentials, by rebounding from hyperpolarization. This raises the question of what the role of excitation is in these neurons.
How This Research Advances What We Know
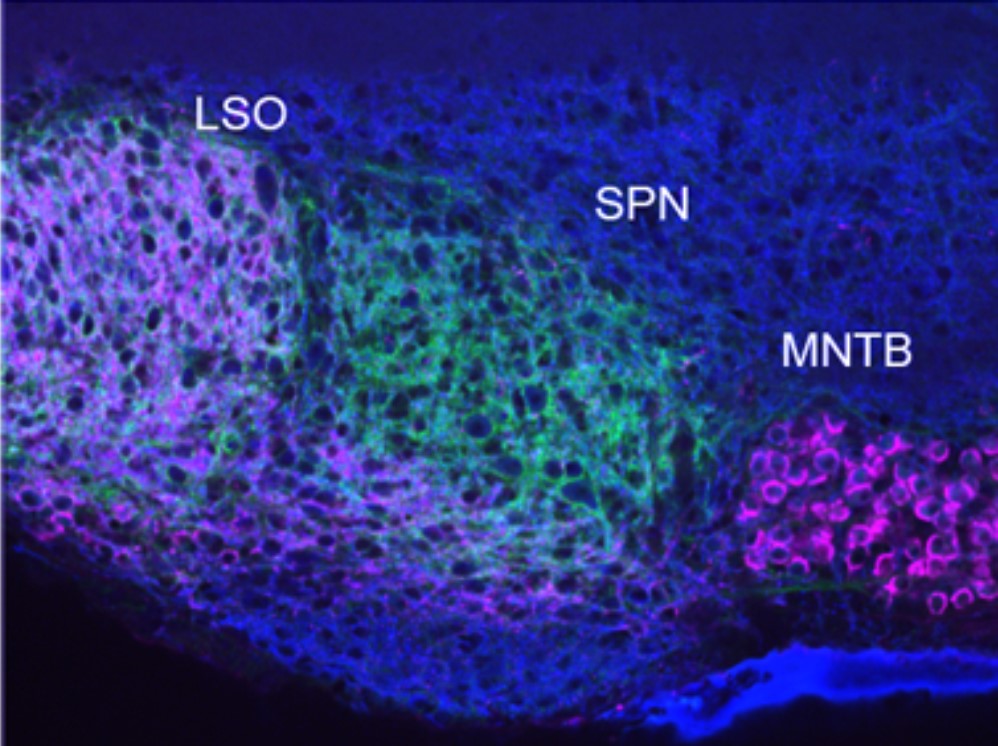
The SPN receives its dominant inhibitory input from the glycinergic medial nucleus of the trapezoid body (MNTB). In vivo recordings show the MNTB fires action potentials during the entire duration of a sound stimulus and essentially inhibits SPN neurons during the sound stimulation. At the end of the sound stimulus, SPN neurons rebound from the aforementioned inhibition to fire action potentials, referred to as offset firing (firing at the offset of sound).
However, in addition to firing at the sound offset, some SPN neurons fire at the beginning of the sound stimulus, referred to as onset firing. This onset firing pattern is reflected by the octopus cells of the posterior ventral cochlear nucleus (PVCN), one source of excitation to SPN neurons.
Based on the inputs, one could infer the onset and offset firing neurons receive more excitation than neurons that only display offset firing. We recorded from SPN neurons in vivo and grouped them into two groups with onset and offset firing neurons as ON-OFF neurons, and neurons with only offset firing pattern as OFF only neurons. We compared the properties of these two groups to gain insight into the role of excitation in SPN neurons.
Experimental Design or Methodology
While characteristic frequency and the sound intensity threshold of the two groups of neurons did not vary, the latency of the offset response was significantly shorter in the ON-OFF group, which received more excitation.
The latency of the offset response depended on the sound intensity of the stimulation, increasing proportionally with the sound intensity. The shorter offset latencies of the ON-OFF neurons are also more stable and don’t change as much as OFF neurons with increasing sound intensities. More excitation renders the ON-OFF neurons to be more precise and reduces their sound level dependency. This made us question if the ON-OFF cells and OFF cells, represent two different subpopulations in the SPN. Does one type receive more excitation?
Results
We used in vitro whole cell recording and immuno-histochemistry to study the synaptic inputs to the SPN. The evidence does not support an anatomical distinction between the two cell populations. We also observed the inhibitory input outweighs the excitation by a factor of ten.
In addition to Alpha-amino 3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, SPN neurons expressed N-Methy-D-aspartate (NMDA) receptors. The slow NMDA current did not depress as much as the inhibitory or AMPA currents, when a train of pulses at 100 Hz was used to stimulate the afferent inputs. This slow excitatory current at the end of a sound stimulus, with moderate depression, could help boost the rebound offset response at the end of inhibition.
Interpretation
Computational modeling simulating ten pulses of excitatory and inhibitory conductance at 100 Hz in the SPN revealed the offset latency at the end of the stimulus is lower in the full model with excitation and inhibition, in comparison to the inhibition only model. It is also noteworthy that the changing the slow NMDA conductance affects the offset latency, while changing the fast AMPA conductance does not.
In vitro whole recording confirmed this effect of excitation serving to shorten offset latency, while simultaneously stimulating inhibitory and excitatory afferent inputs, pharmacologically blocking excitation increases the latency of the offset action potential.
Visit eNeuro to read the original article and explore other content. Read other summaries of JNeurosci and eNeuro papers in the Neuronline collection SfN Journals: Research Article Summaries.
Slow NMDA-Mediated Excitation Accelerates Offset-Response Latencies Generated via a Post-Inhibitory Rebound Mechanism. Ezhilarasan Rajaram, Carina Kaltenbach, Matthew J. Fischl, Leander Mrowka, Olga Alexandrova, Benedikt Grothe, Matthias H. Hennig, and Conny Kopp-Scheinpflug. eNeuro May 2019, 6 (3) 0106–19.2019; DOI: 10.1523/ENEURO.0106-19.2019







