QR2 Modulates Insular Cortex Redox to Enable Novel Taste Memory Formation
Jun 18, 2020
Material below summarizes the article Muscarinic-Dependent miR-182 and QR2 Expression Regulation in the Anterior Insula Enables Novel Taste Learning, published on March 26, 2020, in eNeuro and authored by Nathaniel L. Gould, Alina Elkobi, Efrat Edry, Jonathan Daume, and Kobi Rosenblum.
Highlights
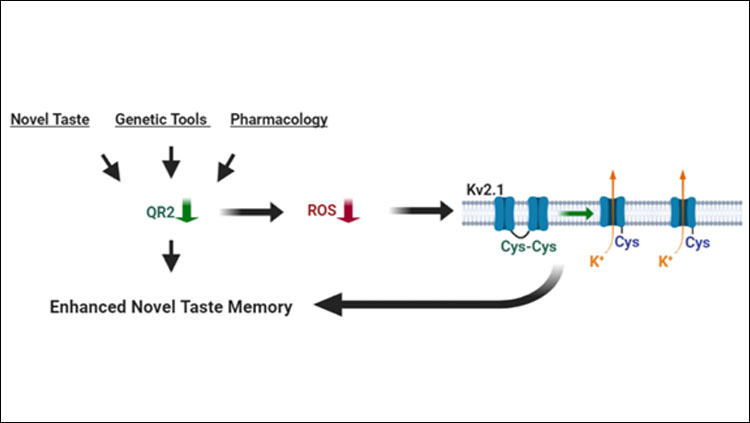
- QR2 is involved across species in the important process of forming new memories of unfamiliar tastes, enabling animals to learn about safe or harmful foodstuffs.
- It does so by being removed from the anterior insular cortex (aIC), via the destabilization of its mRNA by miR182, which is itself dependent on local disinhibition of acetylcholine (ACh) release.
- Removal of QR2 in the aIC reduces reactive oxygen species (ROS) there, affecting the potassium channel Kv2.1, which results in a stable long-term memory of a newly experienced taste, a process that deteriorates with age and disease.
Access to the full article is available to SfN members.
Neuronline is a benefit of SfN membership. Renew your membership now to make sure you don’t lose access.
0 of 5 articles left
Login
or
Become a Member
to unlock content








