Material below summarizes the article An Atoh1-S193A Phospho-Mutant Allele Causes Hearing Deficits and Motor Impairment, published on July 20, 2017, in JNeurosci and authored by Wei Rose Xie, Hsin-I Jen, Michelle L. Seymour, Szu-Ying Yeh, Fred A. Pereira, Andrew K. Groves, Tiemo J. Klisch, and Huda Y. Zoghbi.
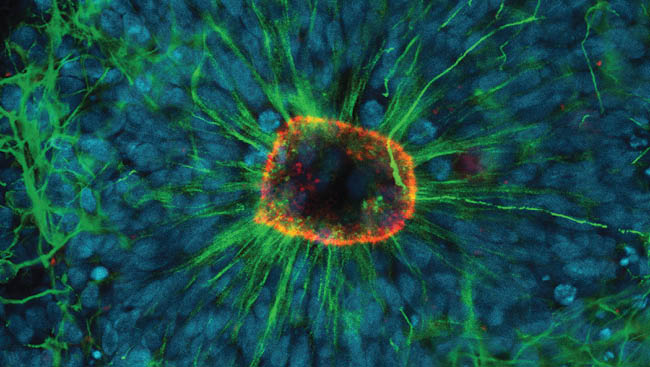
Hair cells of the inner ear play a critical role in the process of hearing. These specialized sensory cells convert mechanical sound waves into an electrical nerve signal that gets sent to the auditory cortex. Many genes contribute to the highly regulated development of these hair cells.
In this study, we discovered that partial loss of one of these genes, Atoh1, causes degeneration of mature inner ear hairs cells and adult hearing loss.
Access to the full article is available to SfN members.
Neuronline is a benefit of SfN membership. Renew your membership now to make sure you don’t lose access.








