New Immunostaining Analysis Reveals Spatial and Molecular Variability in Synaptic Scaling
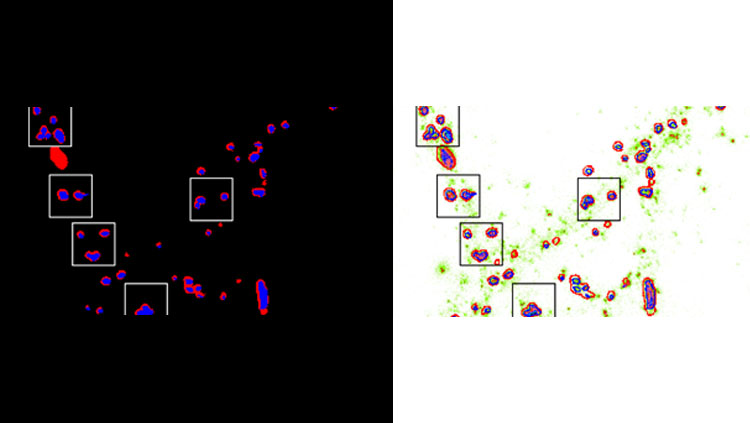
Extracting spatial heterogeneity of AMPA receptor content within functional zones of the synapses to quantify homeostatic scaling. Related to Figure 5 in the manuscript.
Material below summarizes the article Differential Scaling of Synaptic Molecules within Functional Zones of an Excitatory Synapse during Homeostatic Plasticity, published on March 17, 2020, in eNeuro and authored by Sridevi Venkatesan, Sandhya Subramaniam, Premchand Rajeev, Yukti Chopra, Mini Jose, and Deepak Nair.
Highlights:
- A new analytical paradigm allows direct estimation of multiplicative increases in synaptic protein levels during homeostatic scaling in neuronal cultures.
- This method can quantify multiplicative scaling using immunocytochemistry for any pre- or postsynaptic protein within distinct neuronal compartments and could be used in future studies to characterize homeostatic scaling deficits in disease models.
- Together with super-resolution microscopy this analysis revealed that subsynaptic pools of AMPA receptors are altered differentially within functional zones of an excitatory synapse.
Study Question
We sought to devise an analytical paradigm for directly measuring multiplicative scaling of synaptic protein levels using immunocytochemistry to examine spatial heterogeneity in homeostatic synaptic plasticity. We examined whether the total pool of AMPA receptors behaved identically to those localized at the synaptic surface or the postsynaptic density.
How This Research Advances What We Know
Synaptic plasticity is the ability to alter synaptic strength, with changes in molecular and morphological properties of individual synapses in response to variations in neuronal activity. Hebbian plasticity involves synapse strengthening during correlated activation of the presynaptic and postsynaptic neuron. However, such unconstrained positive feedback can destabilize neuronal network activity.
Homeostatic synaptic scaling regulates neuronal excitability following modifications in the network activity by altering all excitatory synapse strengths multiplicatively, to preserve the weight of individual synapses and maintain stable firing.
Chronic suppression of activity in cultured neurons causes a multiplicative increase in EPSC amplitudes indicative of synaptic scaling. However, this characterization is limited to the number of active synapses and underlying molecular and spatial changes remain unknown. We devised a paradigm to quantify scaling from immunostained neuronal cultures. We estimated scaling factors for glutamate receptors and several scaffolding molecules within functional subsynaptic regions, revealing anatomical insights into molecular homeostatic changes.
Methodology
14-day old hippocampal neuronal cultures from neonatal C57B mouse and Sprague Dawley rat pups were incubated with the sodium channel blocker TTX (2μM) for 24 to 48 hours, to induce homeostatic scaling, and then immunostained for various synaptic markers.
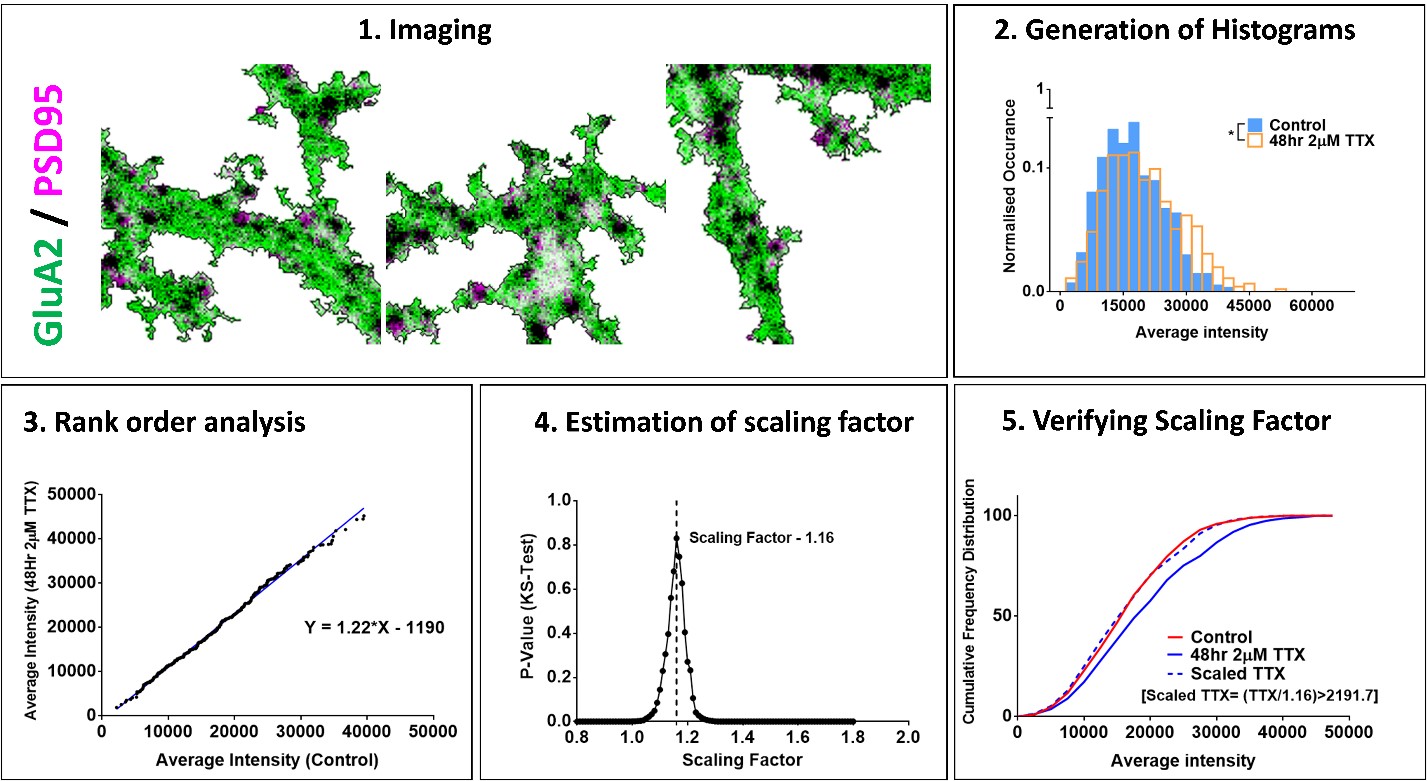
Confocal or super-resolution (STED) microscopy was used to image pyramidal neurons. Average fluorescence intensity and area of synaptic protein puncta were measured using MetaMorph (Molecular Devices) and data were pooled across neurons. A significant difference between control and TTX data distributions indicated scaling.
The slope, as measured from a plot of TTX versus control fluorescence intensities arranged in ascending order and taken from a large set of randomly chosen synapses, gave an estimate for the scaling factor. Hypothetical scaled TTX data were generated by dividing TTX data by a scaling factor. Anderson-Darling (AD) test and/or Kolmogorov-Smirnov (KS) was used to compare this scaled and control distribution to verify the occurrence of synaptic scaling.
 |
|
|
Results
We calculated different scaled TTX data distributions by dividing the TTX data by a range of hypothetical factors and generated a plot of the p-values from KS test comparing each scaled TTX distribution with control data. The scaling factor corresponding to the highest p-value among all comparisons was considered as the most accurate. We repeated this process for 100 different random subsets of all synapses and obtained an average scaling factor.
Using this approach, we determined the scaling factors for synaptic levels of GluA1 and GluA2 AMPA receptors in mouse cultures after 48 hours of TTX treatment, which were of a similar order to previous electrophysiology estimates. Our method could also be reproduced across species and synaptic proteins yielding comparable scaling factors for postsynaptic proteins Shank2, PSD95 and presynaptic Bassoon in mouse and rat cultures.
Longer incubation with TTX (24 versus 48 hours) caused greater scaling up in synaptic levels of surface expressed GluA2, with distal dendrites showing 6 to 7% more scaling than the proximal dendrites. Next, we examined whether the increase in synaptic AMPA receptors during scaling was due to an increase at the PSD or in the adjoining perisynaptic compartment which could serve as a reserve pool to deliver naïve receptors. STED microscopy showed correlated scaling of GluA2 levels at the synapse and perisynapse and increased recruitment of GluA2 to the perisynapse in comparison to the PSD.
Interpretation
Our quantitative method of analyzing immunocytochemical data to estimate scaling factors can be reproduced across species and for any pre- and postsynaptic proteins involved in homeostatic synaptic plasticity. With super-resolution microscopy, we were able to extract scaling factors for AMPA receptors within spatially discrete functional zones of synapses segregated by a few 100nms. We observed greater scaling in the perisynaptic compartment indicating that it could act as a reserve pool of naïve receptors, which would be trafficked in an activity dependent manner to the PSD.
Our study provides an easily adaptable immunocytochemical approach that can be used alongside electrophysiology to obtain anatomical and molecular insights into homeostatic synaptic plasticity. We envision that this would be a useful tool to characterize synaptic scaling deficits in cultures from disease models.
Visit eNeuro to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
Differential Scaling of Synaptic Molecules within Functional Zones of an Excitatory Synapse during Homeostatic Plasticity. Sridevi Venkatesan, Sandhya Subramaniam, Premchand Rajeev, Yukti Chopra, Mini Jose, and Deepak Nair. eNeuro 17 March 2020, 7 (2) ENEURO.0407-19.2020; DOI: 10.1523/ENEURO.0407-19.2020









