Hippocampal CA2 Organizes CA1 Gamma Oscillations During Investigation of Novel Stimuli
Material below summarizes the article Hippocampal CA2 Organizes CA1 Slow and Fast γ Oscillations during Novel Social and Object Interaction, published on March 20, 2020, in eNeuro and authored by Logan Y. Brown, Georgia M. Alexander, Jesse Cushman, and Serena M. Dudek.
Highlights
- CA2 contributes to slow gamma oscillations in CA1 during novel animal investigation and fast gamma during novel animal or novel object investigation.
- Chemogenetic inhibition of CA2 neurons causes a decrease in slow gamma oscillations in the pyramidal cell layer of CA1 selectively during novel animal investigation.
- Chemogenetic inhibition of CA2 causes a layer-specific decrease in CA1 gamma power reflective of CA2’s anatomical inputs to CA1.
 |
|
|
Study Question
Does CA2 contribute to gamma oscillations in CA1 during investigation of a social stimulus relative to a non-social one? Does CA2 organize gamma oscillations in CA1 in a manner reflective of CA2’s anatomical projections to CA1?
How This Research Advances What We Know
Previous studies demonstrated slow gamma oscillations in CA1 rely on CA3 and CA2 input during running, and that CA2 neuronal activity is required for social memory. Whether CA2 neurons coordinate their activity with CA1 when processing stimuli containing socially relevant information, however, remained unexplored.
Given that CA1 gamma oscillations are regulated in a layer-specific manner reflective of its upstream inputs, we predicted that if CA2-dependent oscillations in CA1 contribute to its function in social memory, then they should occur during social exposure and, given the distribution of CA2 axons in CA1, should be organized in a layer-specific manner.
Thus, we sought to answer two questions. First, does CA2 organize CA1 gamma oscillations during investigation of a social stimulus, in addition to during spatial processing? Second, given the pattern of CA2’s anatomical projections in CA1, does CA2 control gamma oscillations in CA1 in a manner reflective of its anatomical connectivity?
Experimental Design or Methodology
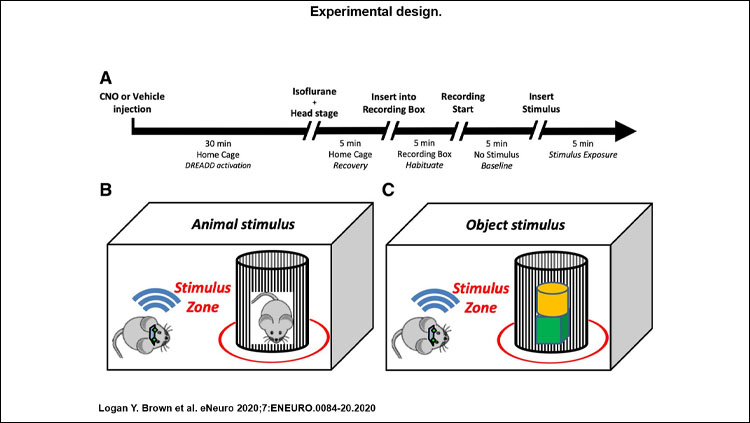
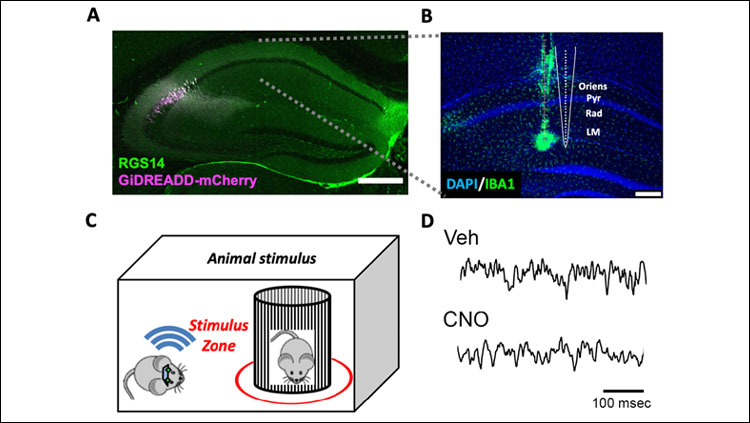
Ten experimental mice expressing cre-recombinase in CA2, and nine control mice were infused with adeno-associated viral vectors carrying inhibitory designer receptors exclusively activated by designer drugs (DREADDs) to allow for selective inhibition of CA2 pyramidal neurons. Subjects were implanted with electrodes to record neural activity from downstream CA1 while subjects freely investigated novel animals or objects.
We then analyzed the recorded local field potentials (LFP) for changes in frequency-specific neural activity. Statistical analysis focused on the theta (6-9 Hz), slow gamma (25-55 Hz), and fast (55-100 Hz) gamma frequency ranges. For statistical analysis of gamma, we used a repeated-measures general linear model to explore potential effects of recording layer, frequency band, stimulus type, and CA2 manipulation via DREADDs.
When significant interactions between experimental variables were detected, we performed repeated-measures, two-tailed, paired t-tests to explore what drove the interaction effect. For analysis of theta power and behavior, we performed paired t-tests.
Results
In this study, we chemogenetically inhibited CA2 pyramidal cells using inhibitory DREADDs to determine whether CA2 contributes to the organization of these oscillations in CA1 during investigation of novel stimuli. We recorded local field potentials from across CA1 layers while subjects freely explored novel juvenile conspecifics or novel inanimate objects and examined neuronal oscillations in the theta, slow gamma, and fast gamma frequency ranges.
We found that CA2 contributes to slow gamma oscillations in CA1 during novel animal investigation and fast gamma during novel animal or object investigation in a layer- and frequency-specific manner reflective of CA2 connectivity with CA1. Inhibition of CA2 caused a significant reduction in slow and fast gamma power in the pyramidal cell layer and stratum oriens, and a reduction of fast gamma power in stratum radiatum. The reduction in slow and fast gamma power was observed only in layers targeted by CA2 pyramidal cells and occurred regardless of whether subjects were investigating a novel animal or a novel object, except in the pyramidal cell layer, where slow gamma was only reduced during investigation of a social stimulus.
No significant effects were detected in stratum lacunosum moleculare, a layer not targeted by CA2 pyramidal cells. Theta oscillations did not significantly differ as a result of any experimental manipulation performed. CA2 inhibition also did not affect the time subjects spent investigating stimuli, or locomotor behavior. Thus, we found that CA1 gamma oscillatory power varies by layer, frequency, and CA2 activity.
Interpretation
These findings reveal three novel points about CA2. First, CA2 organizes gamma oscillations in CA1 during investigation of novel stimuli, besides during running. Second, CA2 modulates gamma activity in the pyramidal cell layer and specific dendritic layers of CA1. Third, CA2 may serve to amplify both slow and fast gamma in CA1.
These findings expand CA2’s role in modulating hippocampal oscillatory networks to include neuronal activity in the fast gamma band, as well as during the repertoire of behaviors CA2 may support. The effect of slow gamma reduction in the pyramidal cell layer only during investigation of a novel social stimulus supports the idea of a role for CA2 in social cognition. The reduction of gamma power in other layers during investigation of objects, however, suggests that the role of CA2 may not be limited to social functions. Instead, CA2 appears to be signaling novelty within hippocampal circuits.
Visit eNeuro to read the original article and explore other content. Read other summaries of eNeuro and JNeurosci papers in the Neuronline collection SfN Journals: Research Article Summaries.
Hippocampal CA2 Organizes CA1 Slow and Fast γ Oscillations during Novel Social and Object Interaction. Logan Y. Brown, Georgia M. Alexander, Jesse Cushman, and Serena M. Dudek. eNeuro 20 March 2020, 7 (2) 0084-20.2020; DOI: 10.1523/ENEURO.0084-20.2020











